A case of "super gonorrhea" has been reported in the United Kingdom, emphasizing warnings from the World Health Organization that drug-resistant superbugs pose a massive public health risk. The WHO is describing the infection as the "worst "ever" case of super gonorrhea. An estimated 78 million people worldwide are infected by gonorrhea every year.
Super gonorrhea is a strain of the bacterial sexually infected infection that has evolved to be resistant to traditional antibiotics. Typically, gonorrhea, known scientifically as Neisseria gonorrhoeae, is treated with azithromycin, ceftriaxone, or spectinomycin, antibiotics that historically have been effective in curing the infection.
But a recent case in the United Kingdom, in which a man infected with gonorrhea failed to respond to conventional treatments, confirms what the World Health Organization has been warning about for years—that antibiotic-resistant superbugs are on the rise. .
According to the WHO, the man became infected after having sexual contact with a woman in southeast Asia.
After conventional treatments using the aforementioned drugs were deemed ineffective in clearing his system of the bacteria, he was placed on ertapenem, an antibiotic typically used to prevent infections following colorectal surgery. Following a total colectomy I had last summer because of a severe case of ulcerative colitis, I was prescribed a course of ertapenem and placed in medical quarantine for three days, having become infected with drug-resistant E. coli.
Swabs of the man's throat confirmed that his strain of gonorrhea was resistant to conventional treatments. The BBC reported this as confirmation of "super gonorrhea."
In July 2017, the WHO reported that out of 77 countries surveyed, 81 percent had documented cases of azithromycin-resistant gonorrhea and a further 66 percent noted a decrease in the effectiveness of ceftriaxone in treating gonorrhea.
The case in the UK, however, is the first confirmed strain of gonorrhea resistant to both antibiotics. In their report, the WHO said that "decreasing condom use, increased urbanization and travel, poor infection detection rates, and inadequate or failed treatment all contribute to this increase."
"The bacteria that cause gonorrhea are particularly smart. Every time we use a new class of antibiotics to treat the infection, the bacteria evolve to resist them," said Dr Teodora Wi, Medical Officer, Human Reproduction, at the WHO. "These cases may just be the tip of the iceberg, since systems to diagnose and report untreatable infections are lacking in lower-income countries where gonorrhea is actually more common."
The WHO also pointed out that more aggressive and accurate testing methods need to be developed and used, and that developing a gonorrhea vaccine should be a priority.
"To control gonorrhea, we need new tools and systems for better prevention, treatment, earlier diagnosis, and more complete tracking and reporting of new infections, antibiotic use, resistance and treatment failures," said Dr Marc Sprenger, Director of Antimicrobial Resistance at WHO. "Specifically, we need new antibiotics, as well as rapid, accurate, point-of-care diagnostic tests – ideally, ones that can predict which antibiotics will work on that particular infection – and longer term, a vaccine to prevent gonorrhea."
Antibiotic resistant superbugs are a result of micro-evolution. Microbes have the ability to mutate and develop their own immunity to drugs that historically have been effective in curing infections, from gonorrhea to certain forms of staph and food-borne pathogens (such as MRSA and E. coli, which are prevalent in hospitals). Antibiotic resistance is due largely in part to over-prescribing antibiotics in both humans and livestock.
The National Institutes of Health issued a report in 2012 confirming this hypothesis, citing strong evidence that the use of antibiotics in meat is directly correlated to the increase of drug-resistant microbes in humans—specifically, the use of antibiotics in otherwise healthy animals.
"Evidence that antibiotic use in food animals can result in antibiotic-resistant infections in humans has existed for several decades. Associations between antibiotic use in food animals and the prevalence of antibiotic-resistant bacteria isolated from those animals have been detected in observational studies as well as in randomized trials. Antibiotic-resistant bacteria of animal origin have been observed in the environment surrounding livestock farming operations, on meat products available for purchase in retail food stores, and as the cause of clinical infections and subclinical colonization in humans."
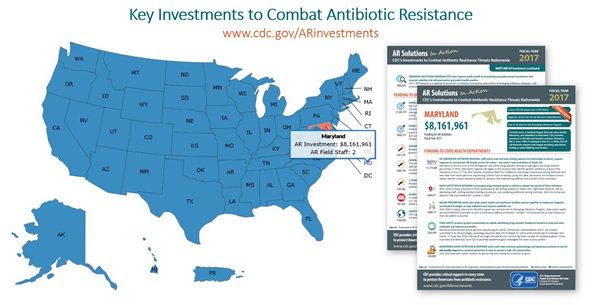
According to the Centers for Disease Control, antibiotic-resistant bacteria claim the lives of more than 23,000 people every year.
Antibiotic / Antimicrobial Resistance | CDC














 Happy Feeling Myself GIF by Laff
Happy Feeling Myself GIF by Laff  Charlie Day Ok GIF
Charlie Day Ok GIF  Happy Sesame Street GIF by Muppet Wiki
Happy Sesame Street GIF by Muppet Wiki 
 @max_balegde/TikTok
@max_balegde/TikTok @max_balegde/TikTok
@max_balegde/TikTok @max_balegde/TikTok
@max_balegde/TikTok @max_balegde/TikTok
@max_balegde/TikTok @max_balegde/TikTok
@max_balegde/TikTok @max_balegde/TikTok
@max_balegde/TikTok @max_balegde/TikTok
@max_balegde/TikTok @max_balegde/TikTok
@max_balegde/TikTok @max_balegde/TikTok
@max_balegde/TikTok @max_balegde/TikTok
@max_balegde/TikTok
 @jackuno/Instagram
@jackuno/Instagram @jackuno/Instagram
@jackuno/Instagram @jackuno/Instagram
@jackuno/Instagram @jackuno/Instagram
@jackuno/Instagram @jackuno/Instagram
@jackuno/Instagram @jackuno/Instagram
@jackuno/Instagram @jackuno/Instagram
@jackuno/Instagram @jackuno/Instagram
@jackuno/Instagram