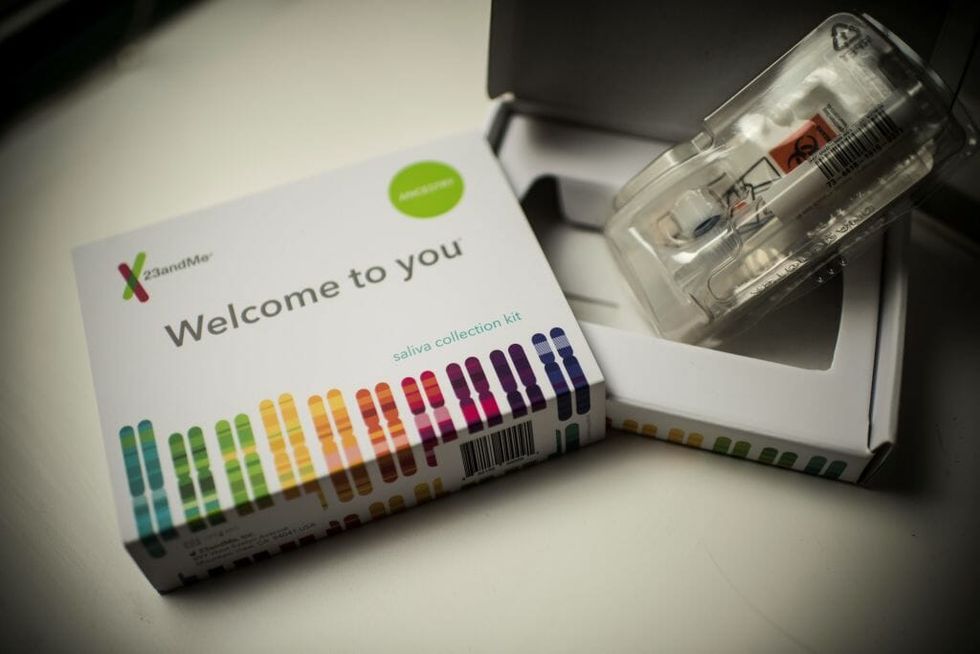
Genetic testing company 23andMe has just gotten FDA approval to sell a test for hereditary colorectal cancer syndrome directly to their consumers.
The test examines three genetic variants of Ashkenazi Jewish ancestry that have indicated a correlation to the cancer syndrome. It's the second time that 23andMe has been approved for a cancer test based on genetic mutations, with the first being a test for the BRCA gene which can act as a litmus test for the likelihood of developing breast cancer.
While the test is innovative in its being sold directly to the consumer, some have concerns.
As with the BRCA tests, some are worried that conducting the tests without medical consultation or supervision could give consumers a false sense of security based on a lack of information and doctoral correspondence. Hereditary colorectal cancer syndrome only accounts for about five percent of inherited colorectal cancer cases, so the test would be far from an assurance of benignity, a fact that may be overlooked by consumers.
Nonetheless, some still find the possibilities promising.
But the company brings with it a host of other concerns.
As mail order DNA testing becomes more prominent, many are concerned about matters of privacy, and the implications of DNA being collected by a private company.
Concerns are especially heightened when factoring in insurance policies.
Regardless of concerns, it doesn't look like the company will stop growing any time soon.







 @NICKIMINAJ/X
@NICKIMINAJ/X





 Red Carpet Picture GIF by Robert E Blackmon
Red Carpet Picture GIF by Robert E Blackmon  Black Friday Christmas GIF by Target
Black Friday Christmas GIF by Target  The Office Boss GIF
The Office Boss GIF  Stay Single Animation Domination GIF by gifnews
Stay Single Animation Domination GIF by gifnews  Season 1 Wedding GIF by NBC
Season 1 Wedding GIF by NBC 
 @kellymengg/TikTok
@kellymengg/TikTok @kellymengg/TikTok
@kellymengg/TikTok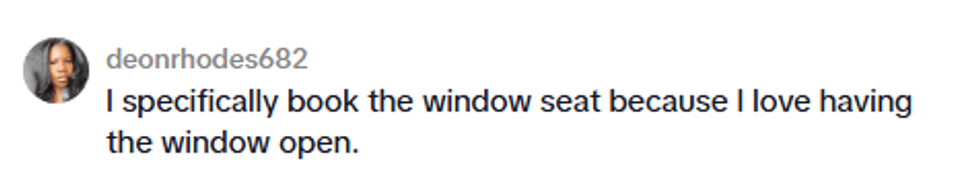 @kellymengg/TikTok
@kellymengg/TikTok @kellymengg/TikTok
@kellymengg/TikTok @kellymengg/TikTok
@kellymengg/TikTok @kellymengg/TikTok
@kellymengg/TikTok @kellymengg/TikTok
@kellymengg/TikTok @kellymengg/TikTok
@kellymengg/TikTok @kellymengg/TikTok
@kellymengg/TikTok @kellymengg/TikTok
@kellymengg/TikTok @kellymengg/TikTok
@kellymengg/TikTok @kellymengg/TikTok
@kellymengg/TikTok @kellymengg/TikTok
@kellymengg/TikTok @kellymengg/TikTok
@kellymengg/TikTok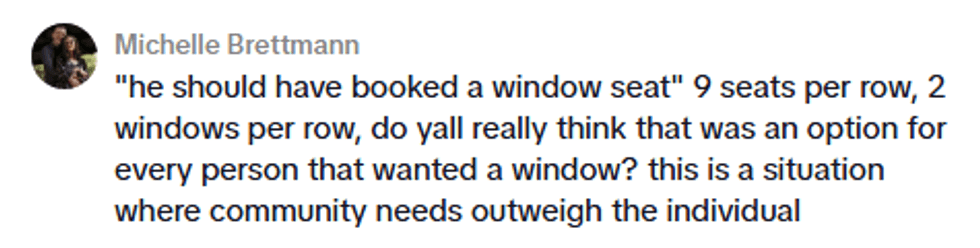 @kellymengg/TikTok
@kellymengg/TikTok
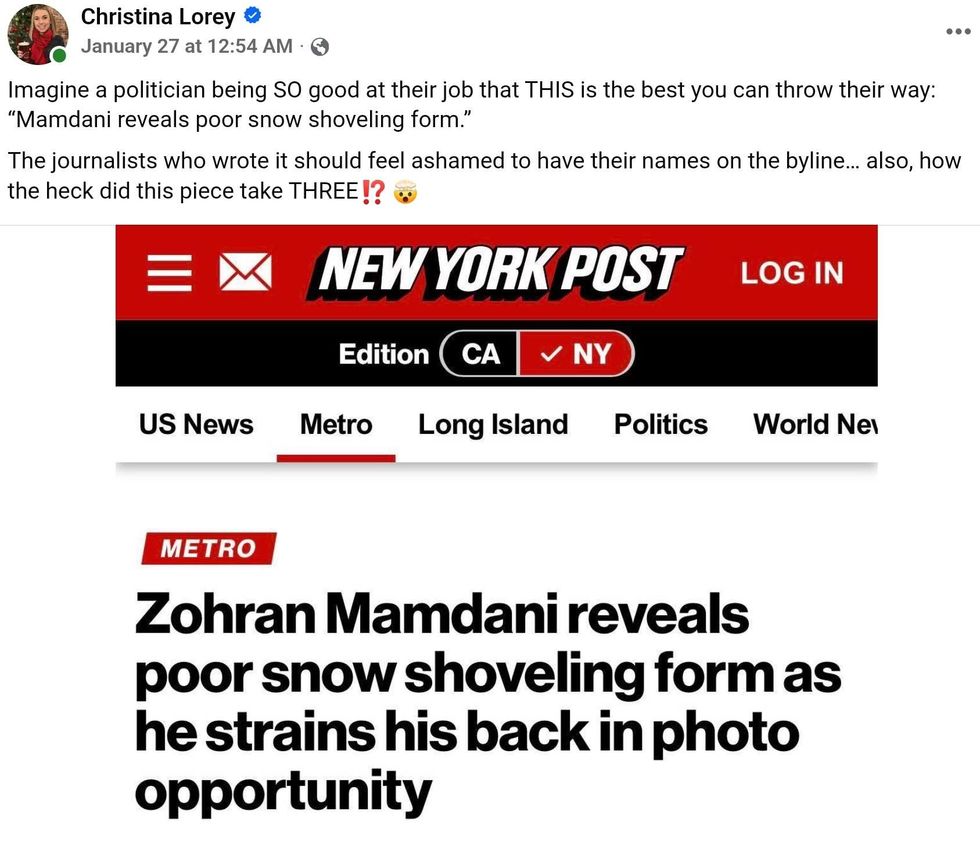 Christina Lorey/Facebook
Christina Lorey/Facebook Barry Novak/Facebook
Barry Novak/Facebook Mary Schroeder/Facebook
Mary Schroeder/Facebook Ben Kayser/Facebook
Ben Kayser/Facebook @danobears/Threads
@danobears/Threads
 @falkthisnonsense/Threads
@falkthisnonsense/Threads @barrypiatoff/Threads
@barrypiatoff/Threads
 @sareineity/TikTok
@sareineity/TikTok @jackb7730/TikTok
@jackb7730/TikTok @theboywanders/TikTok
@theboywanders/TikTok @george199013/TikTok
@george199013/TikTok @mrtempoe/TikTok
@mrtempoe/TikTok @soxfanchris/TikTok
@soxfanchris/TikTok